Photosolubilization of polyolefinsulfone containing
side-chain oxime ester groups
The design and synthesis of new photopolymers has attracted great interest in recent years. The major requirement for photopolymers is that they exhibit a chemical change that allows the irradiated parts of the polymer to be distinguished from the nonirradiated parts, usually on the basis of solubility.1 A UV-induced change in the polarity of a polymer enhances the solubility of the irradiated parts. There have been many reports of polymer photosolubilization in which the polarity of polymers has been changed by photochemical means. Photobase-generating polymers are one such type. Photopolymers bearing photobase-generating groups are expected to be particularly applicable in photolithographic processes because their amino groups do not corrode metals.
Polyolefinsulfones contain sulfonyl groups (-SO2-) in the main chain. Their practical applications include use in engineering plastics and in functional polymers such as heat-resistant polymers, adhesives, and electron beam resists.2,3 As the sulfonyl group is a strong electron-withdrawing moiety, the neighboring methylene becomes acidic, so that the protons on the methylene can be extracted by bases, which leads to main-chain scission. In this way, the addition of a base such as amine to a polyolefinsulfone solution gives rise to decomposition of the polymer. Thus, a polyolefinsulfone containing a photobase generator has potential as a photodecomposition polymer. In this study, a polyolefinsulfone possessing a photobase-generating moiety in the side chain (polyacetophenone-3-butenoyloxime-sulfone) was synthesized (Figure 1), and photosolubilization of the polymer was examined. An oxime ester was chosen as the photobase-generating group;4-12 when exposed to 365-nm light, this group is photochemically converted into an imino group, which can be changed to an amino group by adding the polymer to a dilute HCl solution (Figure 1).
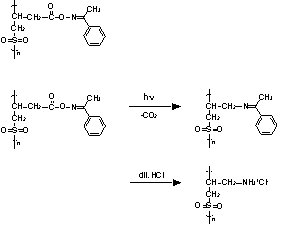
Figure 1. Structure of polyolefinsulfone bearing an oxime ester group (ABO-SO2 polymer), and scheme of the photoreaction.
Acetophenone-3-butenoyloxime (ABO monomer) was synthesized by reaction of acetophenone oxime with 3-butenoic chloride, and then polymerized in liquefied sulfur dioxide at -13°C by radical polymerization.13 Tert-butyl hydroperoxide was used as a redox initiator. Because the stability of the sulfonyl radical is much higher than that of the vinyl radical, and the sulfur atom is positively charged, a 1:1 alternating copolymer of SO2 and the olefin monomer is obtained. The copolymerization ratio was examined by elemental analysis.
A chloroform solution of ABO-SO2 polymer and a sensitizer (benzophenone, 10 wt% relative to the polymer) was spin-coated on a glass plate. The thickness of the film was 1 ?m. The sample film was irradiated by a 250-W super high-pressure mercury lamp with an interference filter (365 nm, 1 mW/cm2). After irradiation, the film was immersed in 0.012 N HCl for 10 min at 80°C.
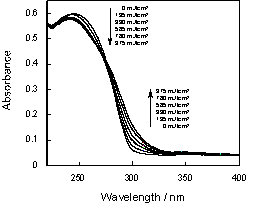
Figure 2. UV absorption spectra of 1-micro meter thick ABO-SO2 polymer film with irradiation at 254 nm
Figure 2 shows the UV-vis absorption spectra of ABO-SO2 polymer film before and after irradiation. It can be seen that the absorption around 250 nm decreases with irradiation, while that of the imino group (300 nm) increases. The photochemical reaction of the oxime ester group was examined by IR absorption measurement (Figure 3, inset). A decrease in absorbance at 1756 cm-1 (C=O stretching) demonstrated photoinduced decarboxylation of the oxime ester group. The fraction of oxime ester groups remaining after irradiation is plotted as a function of irradiation energy in Figure 3. A total of 60% of oxime ester groups were converted to imino groups by irradiation of 3500 mJ/cm2.
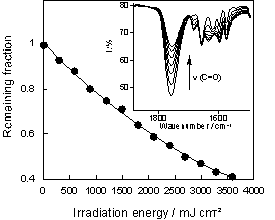
Figure 3. Remaining fraction of oxime ester groups as a function of irradiation energy.
Inset shows the IR absorption spectra of the film before and after irradiation.
Photosolubilization of the ABO-SO2 polymer was also examined. After irradiation,
the ABO-SO2 polymer film was immersed in 0.012 N HCl. The film thickness
was measured by atomic force microscopy (AFM); normalized film thickness
is plotted as a function of irradiation energy in Figure 4.
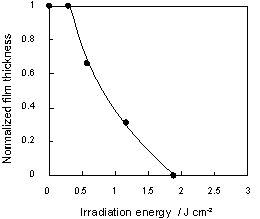
Figure 4. Thickness of ABO-SO2 film after immersion in 0.012 N HCl at 80°C for 10 min.
A 0.5-?m film of ABO-SO2 polymer was completely solubilized in 0.012 N HCl solution by 365 nm irradiation of 1900 mJ/cm2. An ABO-SO2 polymer film was irradiated with a photomask and a photopattern was developed; Figure 5 shows a SEM image of the irradiated area. It can be seen that the irradiated area was completely dissolved, creating a sharp contrast with the non-irradiated area. This indicates that the ABO-SO2 polymer functions as a positive photoimaging polymer.
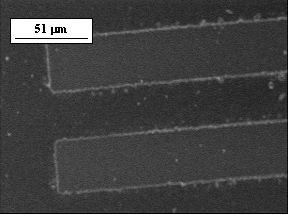
Figure 5. SEM image of micropattern formed on the ABO-SO2 polymer film.
Publications
Photoinduced Depolymerization in Poly(olefin sulfone) Films Composed of Volatile
Monomers Doped with a Photobase Generator
T. Sasaki, T. Kondo, M.
Noro, K. Saida, H. Yaguchi and Y. Naka
.J. Polym. Sci. Part A: Polym.
Chem., 50, 1462-1468 (2012).
Inside Cover Article
Photoinduced Depolymerization of Poly(olefin sulfone)s Possessing Base
Amplifying Groups
T. Sasaki, H. Yaguchi,
J. Polym. Sci. Part A:
Polym. Chem., 47, 602-613 (2009).
Photoinduced Depolymerization of Poly(olefin sulfone)s Possessing Photo-base
Generating Groups in the Side-chain
H. Yaguchi, and T.
Sasaki
Macromolecules, 40, 9332-9338 (2007).
Photosolubilization of Polyolefinsulfone Possessing Side Chain Oxime Ester
Groups
H. Yaguchi, and T. Sasaki
Chem. Lett. 35, 760-761
(2006).
photopolymer photopolymer photopolymer photopolymer photopolymer photopolymer
photopolymer photopolymer
photopolymer photopolymer photopolymer photopolymer photopolymer photopolymer
photopolymer photopolymer
photopolymer photopolymer photopolymer photopolymer photopolymer photopolymer photopolymer photopolymer
photopolymer photopolymer photopolymer photopolymer photopolymer photopolymer
photopolymer photopolymer
photopolymer photopolymer photopolymer photopolymer photopolymer photopolymer
photopolymer photopolymer
photopolymer photopolymer photopolymer photopolymer photopolymer photopolymer
photopolymer photopolymer