Photorefractive Effect of LC Polymers Containing Hydrogen-Bonding Moiety
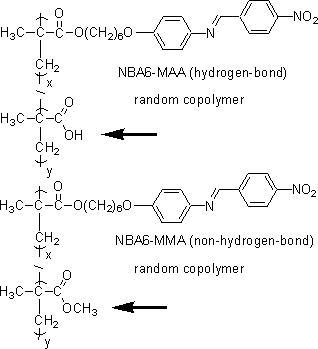
Figure 1
The enhancement of photorefractivity in LC polymers is considered to arise
from the microscopically-ordered structure of component chromophores in
the isotropic phase. If the microscopically-ordered structure plays an
important role in the enhancement of the photorefractive effect, the introduction
of a hydrogen-bonding moiety into the polymer would significantly affect
the photorefractivity. To investigate this possibility, the photorefractivity
of D-pai-A polymers that possess a hydrogen-bond-formable moiety was compared
with that of polymers without hydrogen-bonding moieties.35 The photorefractivity
of copolymers of a nitrobenazylideneaniline monomer and methacrylic acid
(NBA6-MAA) were investigated. The carboxylic acid moieties are capable
of forming hydrogen-bonds and this introduces micro-domains in the polymer
film. Copolymers of nitrobenzylidenaniline monomer and methyl methacrylate
(NBA6-MMA) were used as reference polymers. The glass transition temperature
and nematic-isotropic phase transition temperature are both higher in NBA6-MAA
compared to NBA6-MMA. Conformational changes of the main chain of NBA6-MAA
are restricted by the hydrogen-bonding and could lead to higher phase transition
temperatures. The temperature dependence of the diffraction efficiencies
of NBA6-MAA and NBA6-MMA mixed with 30 wt% CDH and 1 wt% TNF are shown
in Figure 2. The diffraction efficiency of the non-hydrogen-bonding polymer
NBA6-MMA increased with raising temperature around Tg and decreased at
higher temperatures. However, the hydrogen-bonding polymer NBA6-MAA exhibited
larger diffraction efficiencies at temperatures below Tg which decreased
as the temperature was raised above Tg. For the copolymerization ratios
considered, the diffraction efficiencies of NBA6-MAA at temperatures T/Tg
= 0.7 ~ 0.9 were much larger than those of NBA6-MMA. The larger diffraction
efficiencies in NBA6-MAA are considered to originate from the large mobility
of the side-chain D-pai-A chromophores below Tg and due to an enhancement
in the electro-optic effect caused by the presence of micro-domains. A
change in the refractive index for the photorefractive effect in organic
polymers arises chiefly from the orientational change of D-pai-A chromophores.
However, in common photorefractive polymers, the mobility of side-chain
D-pai-A chromophores in the film is low at temperatures below Tg. It was
considered that the mobility of D-pai-A chromophores in NBA6-MAA films
at temperatures below Tg was enhanced by the presence of the hydrogen-bonding.
The conformation of the main-chain of NBA6-MAA is restricted by the hydrogen-bonding
and is likely to be in a stressed state. This results in a larger inner-free-volume
around the chromophores as shown in Figure 3. The glass transition temperature,
below which the main chains freeze, was higher in NBA6-MAA. However, the
free volumes around the side-chains likely swelled because of the stressed
conformation of the main-chains. When the temperature was raised above
Tg, the hydrogen-bonding was broken and the conformation of the main-chain
relaxed to the thermally stable state. The side-chains are closely packed
and this leads to smaller free volumes around the D-pai-A chromophores.
Moreover, the microdomains formed via the hydrogen-bonding are disorganized
at temperatures above Tg and this lowers the photorefractivity.
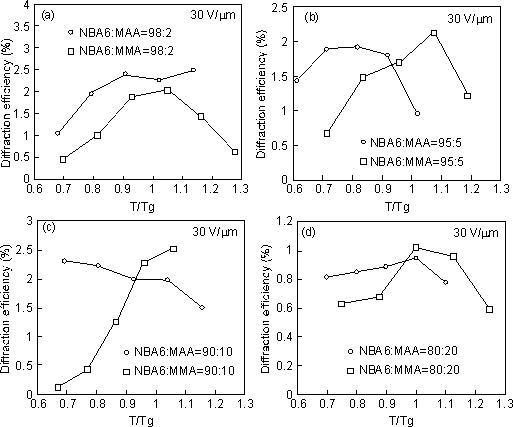
Figure 2
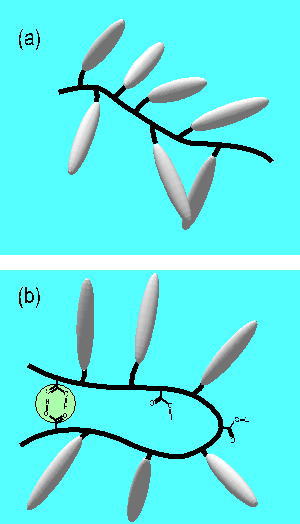
Figure 3
T. Sasaki, G. Fukunaga, Chem. Mater., 17, 3433-3438 (2005).