| Contents | Members | Åß̤ | Pubulications | |
| Recent Research | (Originals) | (Reviews) | ||
| Recent Research (Page 2) |
| Back to Page 1@¨ |
| Download Page 2 |
| Research from Soaifs group |
| Asymmetric Autocatalysis and the Origin of Homochirality of Biomolecules |
| Kenso SOAI |
| Department of Applied Chemistry, Tokyo University of Science, Kagurazaka, Shinjuku-ku, Tokyo 162-8601 e-mail: soai@rs.tus.ac.jp |
| Introduction |
| It is well known that life on the Earth is composed of L-amino acids and D-sugars. Like left and right hands, L-amino acid and D-amino acids are mirror images and are not superimposable (Fig. 1). They are termed as enantiomers and are chiral. One of the most characteristic features of life is that many biomolecules are highly enantioenriched enantiomers. If proteins ad enzymes contain D-amino acids in random manner, the proteins and enzymes will not operate in normal manner. If DNA and RNA contain L-sugars in a random manner, the normal helical structure will not be formed and DNA and RNA will not work. In usual chemical reactions, the probability of the formation of D and L enantiomers is one to one, and the products are recemic modifications which contain one to one ration of D and L enantiomers. In sharp contrast, all of the living organisms on the Earth are composed of only L-amino acids. In addition, DNA and RNA of all living organisms employ only D-sugars. The chiral homogeneity of biomolecules, i.e., homochirality, is one of the striking features of life and is considered to be closely related to the origin of life. |
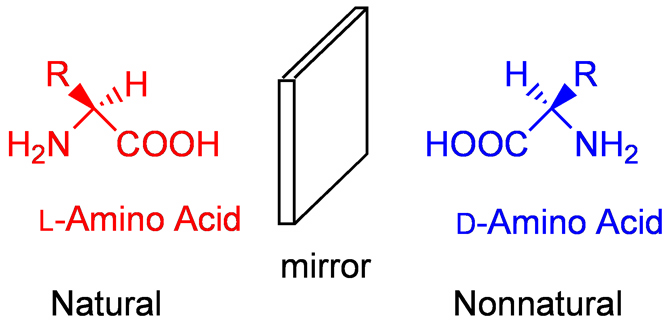 |
| Fig.P Natural L-amino acid and its enantiomer |
| Why, when and how did biomolecules become highly enantiomerically enriched? What is the origin of chirality? It has been considered that, on the early Earth or in space, by some reason, the initial racemic mixture of D and L enantiomers had become highly enantioenriched. Was it possible for such a reaction to really occur? Several theories have been proposed as the origins of chirality of organic compounds; right and left circularly polarized light (CPL), chiral inorganic crystals such as d and l-quartz, d and l-sodium chlorate, statistical fluctuation (spontaneous generation). However, the enanriomeric imbalances induced by these mechanisms have been very low (less than 2% enantiomeric excess). Therefore, in order to reach the high enantioenrichment observed in nature, the process has been needed for significant amplification of extremely low enantiomeric excess to very high enantiomeric excess. |
| We discovered asymmetric autocatalysis in which chiral product acts as chiral catalyst for its own production with significant amplification of enantiomeric excess. Moreover, we found asymmetric autocatalysis using chiral quartz and right and left circularly polarized light as the origins of chirality. In addition, we reported the first experimental example of a spontaneous absolute asymmetric synthesis by using asymmetric autocatalysis with amplification of chirality [1]. |
| Discovery of Asymmetric autocatalysis |
| Asymmetric autocatalysis, i.e., asymmetric automultiplication and catalytic replication of chiral compound, is a reaction in which the structures of chiral catalyst and the product are the same and in which chiral product acts as a chiral catalyst for its own production (Fig. 2). In usual asymmetric catalysis, because the structures of chiral catalyst and the product are different, it is necessary to separate the product from the catalyst. In addition, because the amount of catalyst doesnft increase during the reaction, the amount of the recovered catalyst decreases and the catalytic activity intrinsically decrease. |
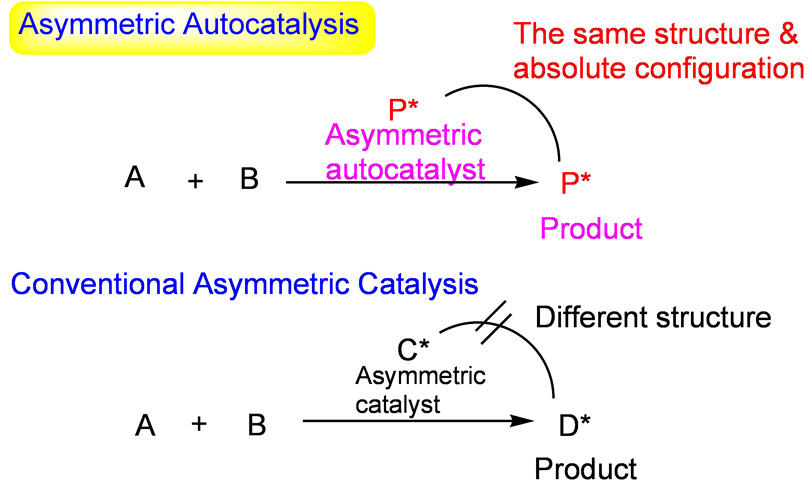 |
| Fig. 2 Asymmetric autocatalysis and conventional asymmetric catalysis |
| Unlike usual asymmetric catalysis, asymmetric autocatalysis has the following advantages: Because the structures of catalyst and the product are the same, itfs not necessary to separate the product from the catalyst. The efficiency of the reaction is high, because the amount of the catalyst increases during the reaction. The decrease in the amount and the catalytic activity of chiral catalyst can be avoided. In asymmetric autocatalysis, it can be anticipated that the initial tiny imbalance of chirality can be amplified without the need of any other chiral compound. |
| We found an asymmetric autocatalysis of pyridyl alkanol [2] and pyrimidyl alkanol [3]; pyrimidyl alkanol asts as an asymmetric autocatalyst in the enantioselective addition of diisopropylzinc to pyrimidine-5-carbaldehyde to afford the same pyrimidyl alkanol of the same absolute congifuration with the initial asymmetric autocatalyst (Fig. 3) [3]. The reaction is an automultiplication of enantioenriched pyrimidyl alkanol. Near perfect asymmetric autocatalysis with greater than 99.5% enantiomeric excess has been achieved [4]. When the product, i.e., pyrimidyl alkanol, is used as an asymmetric autocatalyst for the next round, the enantiomeric excess is greater than 99.5%. Even after 10th round, the enantioselectivity of product & catalyst is greater than 99.5%, only one enantiomer has automultiplied in near quantitative yield. The result shows that, during the 10 consecutive rounds of asymmetric autocatalysis, the initial enantiomer of pyrimidyl alkanol has automultiplied by a factor of 60 million times. |
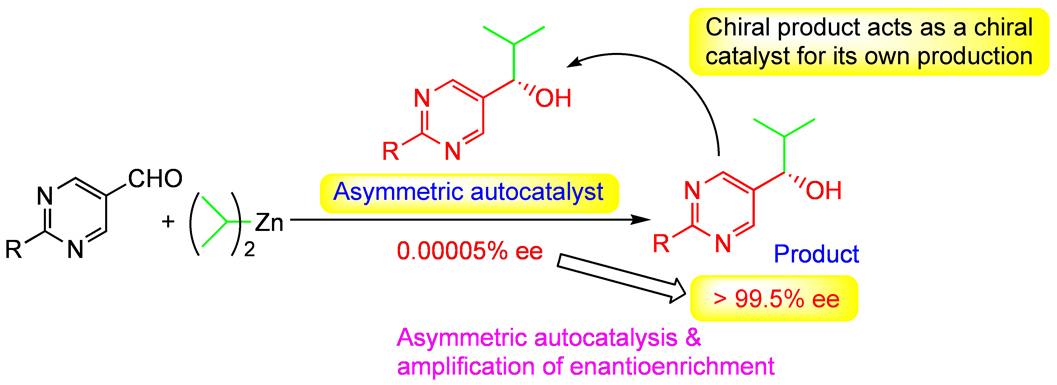 |
| Fig. 3@Asymmetric autocatalysis with amplification of chirality |
| Asymmetric Autocatalysis with Amplification of Chirality |
| When pyrimidyl alkanol with low enantiomeric excess was employed as an asymmetric autocatalysis, we found that the enantioenrichment of the product, i.e., pyrimidyl alkanol is amplified (Fig. 3) [2a]. To take advantage of asymmetric autocatalysis that the structures of chiral catalyst and the product are the same, consecutive asymmetric autocatalysis was carried out by using the product of one round as asymmetric autocatalyst for the next round. Starting from extremely low enantiomeric excess (about 0.00005% ee, this corresponds to the difference of only several number of molecules between ca. 5 million molecules of R enantiomer and 5 million molecules of S enantiomer) of (S)-pyrimidyl alkanol, three consecutive asymmetric autocatalyses afford near enantiopure pyrimidyl alkanol with greater than 99.5% enantiomeric excess [5]. During these three consecutive asymmetric autocatalysis, the initial slightly predominant enantiomer of (S)-pyrimidyl alkanol has automultiplies by a factor of 630,000 times, while the initial slightly minor enantiomer of (R)-pyrimidyl alkanol less than one thousand. As described, we have found asymmetric autocatalysis in which slight enantiomeric imbalance in chirality is amplified significantly |
| Research on the Origin of Chirality of organic compounds by Using Asymmetric Autocatalysis |
| Asymmetric autocatalysis can be applied for the examination of the proposed origins of chirality (Fig. 4). |
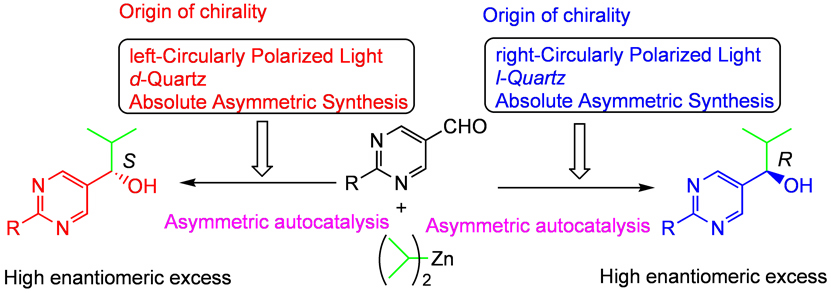 |
| Fig. 4 Asymmetric autocatalysis and the origin of chirality |
| 1. Right and Left Circularly Polarized Light |
| Circularly polarized light (CPL) exhibits right or left handedness and is a chiral physical force. It is known that CPL induces tiny enantiomeric imbalances in some of the organic compounds but the enantiomeric excess is very low: it is reported that photodecomposition of racemic leucine by irradiating right CPL gives slightly enantioenriched (less than 2% ee) L-leucine. |
| We found that asymmetric autocatalysis can correlate the low enantioenrichment of the obtained leucune with the high enantioenrichment of organic compound. Thus, in the presence of L-leucine with low (2%) enantioenrichment, the reaction between pyrimidine-5-carbaldehyde and diisopropylzinc and the subsequent asymmetric autocatalysis afforded highly enantioenriched pyrimidyl alkanol with the correlated absolute configuration with amino acid [6]. The initially formed pyrimidyl alkanol has tiny enantiomeric excess owing to the presence of enantioenriched leucine, and the subsequent asymmetric autocatalysis with amplification of chirality affords the highly enantioenriched pyrimidyl alkanol. It is reported that strong CPL occurs in the star formation region in space. |
|
Direct irradiation of left-CPL (wavelength: 313 nm) to racemic (mixture
of R and S enantiomers with the ratio of 50:50) pyrimidyl alkanol, and
the subsequent asymmetric autocatalysis affords (S)-pyrimidyl alkanol with
greater than 99.5% enantiomeric excess [7]. On the contrary, the irradiation
with right-CPL (313 nm) and the asymmetric autocatalysis gave (R)-pyrimidyl
alkanol with greater than 99.5% enantiomeric excess. As described, asymmetric autocatalysis enables circularly polarized light (CPL) to play a role of the origin of chirality of organic compounds with very high enantiomeric excess. |
| 2. Chiral Inorganic Crystals such as d and l-Quartz |
| Quartz occurs naturally and exhibits d and l-enantiomorphs. It has been considered that quartz is the origin of chirality of organic compounds. However, the enantiomeric excess induced by quartz has been extremely low and no experiment has been report which correlates the chirality of quartz and that of organic compound with high enantiomeric excess. We found that, in the presence of the powder of d-quartz, the reaction between pyrimidine-5-carbaldehyde and diisopropylzinc and the subsequent asymmetric autocatalysis affords (S)-pyrimidyl alkanol with very high enantiomeric excess, while in the presence of l-quartz (R)-alkanol with very high enantiomeric excess was formed (Fig. 4) [8]. Thus, an organic compound with high enantiomeric excess is synthesized for the first time, in conjunction with asymmetric autocatalysis, using quartz as the origin of chirality. We also found that d and l-sodium chlorate serves as the chiral initiator of asymmetric autocatalysis [9]. |
| As described, asymmetric autocatalysis with amplification of chirality afford, for the first time, the highly enantioenriched organic compound using quartz as the origin of chirality. |
| 3. Spontaneous Absolute Asymmetric Synthesis |
| It has been well accepted that, without the intervention of any chiral
factor, the probability of the formation of R and S product is fifty to
fifty (50 : 50); racemate is formed. However, according to the theory of
statistics, the numbers of R and S enantiomers are not exactly the same,
i.e., there is almost always the fluctuation in numbers of enantiomers.
Letfs think about tossing coin in 100 times: the probability is only 0.08
(8 times) that you get exactly 50 heads and 50 tails in 100 times trials.
In the rest of 0.92 probability (92 times), the numbers of head and tail
are not the same. Moreover, when the total number is an odd number, you
cannot get exactly the same number of head and tail. Like toss the coins,
one can consider the initial fluctuation of chirality in organic reaction
without the intervention of any chiral factor. Of course, when the number
of molecules becomes large, the enantiomeric excess is below the detection
level. As we described before, asymmetric autocatalysis of pyrimidyl alkanol has enormous power of amplification of enantiomeric excess from extremely low (about 0.00005% ee) to greater than 99.5% enantiomeric excess [5]. |
| We found that the reaction of pyrimidine-5-carbaldehyde with diisopropylzinc, without adding any chiral material, gives enantioenriched pyrimidyl alkanol with either R or S absolute configurations (Fig. 4) [10]. The formation of R and S pyrimidyl alkanol exhibits approximate stochastic 1 to 1 distribution. It is conceivable that the initially formed stochastic fluctuation in chirality is amplified by asymmetric autocatalysis. The results of stochastic formation of R and S pyrimidyl alkanoil fulfill the conditions necessary for spontaneous absolute asymmetric synthesis. This is the first example of a spontaneous absolute asymmetric synthesis. We believe this has significant implication in the origin and amplification of chirality in nature. |
| 4. Asymmetric Autocatalysis Initiated by Chiral Crystals fromed from Achiral Organic Compounds |
| Some of the achiral organic compounds form chiral crystals. However, these chiral crystals have not been known to induce chirality in other compounds. We found that asymmetric autocatalysis is initiated by these chiral crystals formed from achiral organic compounds such as co-crystals and N-benzoylglycine (hippuric acid) to afford highly enantioenriched pyrimidyl alkanol with the corresponding absolute configurations with those of chiral organic crystals [11]. Thus, by using asymmetric autocatalysis, the formation of chiral organic crystals from achiral organic compounds has been successfully correlated to high enantioenrichments of other organic compound. |
| References |
|
[1] Reviews. (a) K. Soai, T. Shibata and I. Sato, Acc. Chem. Res., 2000,
33, 382-390. (b) K. Soai and T. Kawasaki, Chirality, 2006, 18, 469-478. (c) K. Soai, gAsymmetric Autocatalysis, Absolute Asymmetric Synthesis and Origin of Homochirality of Biomolecules,h in gProgress in Biological Chirality,h Ed. by G. Palyi, C. Zucchi, Chap. 29, pp. 355-364, Elsevier, Oxford, 2004. [2] K. Soai, S. Niwa, and H. Hori, J. Chem. Soc., Chem. Commun., 1990, 982-983. [3] (a) K. Soai, T. Shibata, H. Morioka and K. Choji, Nature (London), 1995, 378, 767-768. (b) T. Shibata, H. Morioka, T. Hayase, K. Choji and K. Soai, J. Am. Chem. Soc., 1996, 118, 471-472. [4] T. Shibata, S. Yonekubo and K. Soai, Angew. Chem. Int. Ed., 1999, 38, 659-661. [5] I. Sato, H. Urabe, S. Ishiguro, T. Shibata and K. Soai, Angew. Chem. Int. Ed., 2003, 42, 315-317. [6] T. Shibata, J. Yamamoto, N. Matsumoto, S. Yonekubo, S. Osanai and K. Soai, J. Am. Chem. Soc., 1998, 120, 12157-12158. [7] T. Kawasaki, M. Sato, S. Ishiguro, T. Saito, Y. Morishita, I. Sato, H. Nishino, Y. Inoue, K. Soai, J. Am. Chem. Soc., 2005, 127, 3274-3275. [8] K. Soai, S. Osanai, K. Kadowaki, S. Yonekubo, T. Shibata and I.Sato, J. Am. Chem. Soc., 1999, 121, 11235-11236. [9] I. Sato, K. Kadowaki and K. Soai, Angew. Chem. Int. Ed., 2000, 39, 1510-1512. [10] (a) (Japanese patent) K. Soai, T. Shibata and Y. Kowata, gAsymmetric Synthesis of Enantioenriched Alkanol by Spontaneous Asymmetric Synthesis,h Japan Kokai Tokkyo Koho, JP 1997, 9-268179. Application date: February 1 and April 18, 1996. An abstract of JP 9268179 is available from the European Patent Office. (http://ep.espacenet.com). (b) K.Soai, I. Sato, T. Shibata, S. Komiya, M. Hayashi, Y. Matsueda, H. Imamura, T. Hayase, H. Morioka, H. Tabira, J.Yamamoto and Y. Kowata, Tetrahedron Asymmetry, 2003, 14, 185-188. (c) T. Kawasaki, K. Suzuki, M. Shimizu, K. Ishikawa, K. Soai, Chirality, 2006, 18, 479-482. [11] (a) T. Kawasaki, K. Jo, H. Igarashi, I. Sato, M. Nagano, H. Koshima, K. Soai, Angew. Chem. Int. Ed., 2005, 44, 2774-2777. (b) T. Kawasaki, K. Suzuki, K. Hatase, M. Otsuka, H. Koshima, K. Soai, Chem. Commun., 2006, 1869-1871. |
|
Download PDF file of Page 2 |
| Back to Page 1@¨ |