Research
For next-generation energy conversion!
To resolve environmental and energy issues, new materials with higher chemical/electric energy conversion efficiency are required.
We are synthesizing new electrode materials and investigating the charge/discharge mechanisms to achieve high performance Lithium-ion batteries and capacitor, and the practical use of Sodium, Potassium-ion batteries, aiming High-energy, long-life, and environmentally friendly batteries for next generation devices and vehicles.
Furthermore, we are researching the electrochemical sensor which detects the specific components selectively from electric signal, and bio-fuel cells genrating electric power by using enzyme reactions at electrode.
We are promoting research and developments to apply chemistry to daily life through collaborative study with companies on basic research.
Research (PDF)
Lithium-ion battery
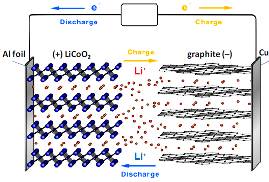
Nowadays, Lithium-ion batteries are widely used for electric power source of not only portable devices such as mobile phone, smart phone or laptop, but vehicles, and become essential for our daily lives. We try to develop new materials for high performance Lithium-ion batteries.
Sodium-ion battery
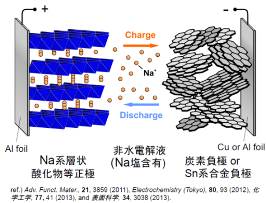
Li resource for Lithium-ion battery is unevenly distributed to plitically instable South America region, and disturbance of stable supply is worried. For that reason, Sodium-ion battery using Na, which is an element just next to Li in the periodic table of elements, attracts attention and is studied by researches. Sodium-ion battery is expected realizing low-cost, much environmental benign, and large capacity comparable to current Lithium-ion battery.
Potassium-ion battery
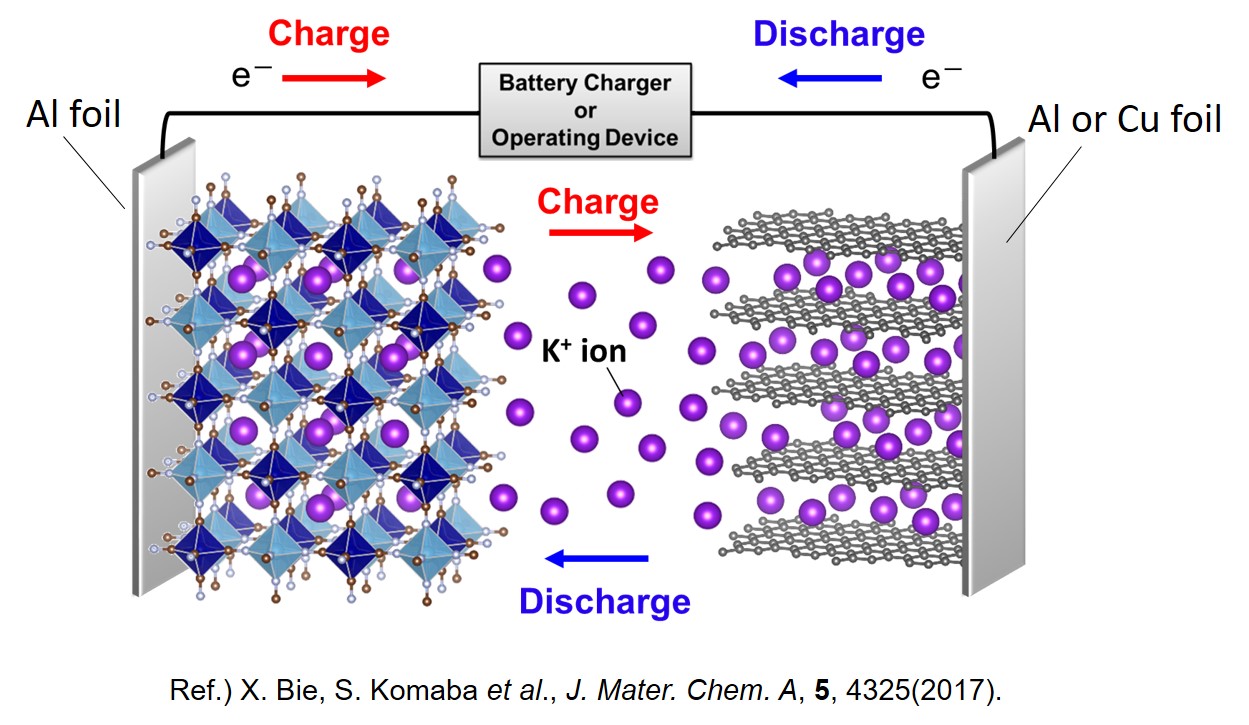
For the alternative of Lithium-ion battery following Sodium-ion battery, we focus on Potassium-ion battery which uses K+ ion as ionic carrier, earth abundant monovalent cation as Li+ and Na+. Potassium-ion battery is anticipated enabling high energy density by high operating voltage originating from lower standard electrode potential and rapid charge/discharge by lower Lewis acidity of K+ ion than Li+ and Na+ ions.
Supercapacitor
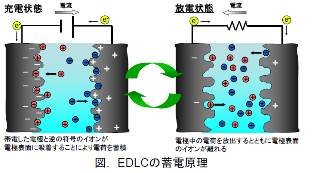
Erectrochemical capacitor can materialize faster charge/discharge and longer cycle life than those of rechargeable batteries. Electrochemical capacitor is categolized into "Electric Double Layer Capacitor(EDLC)" and "Redox Capacitor" by the differences in principle of charging. EDLC gathers electric charges by using electric double layer which is formed at the interface between electrode and electrolyte. On the other hand, Redox Capacitor has larger capacity than EDLC by combining electric double layer and redox reaction.
Bio-fuel cell
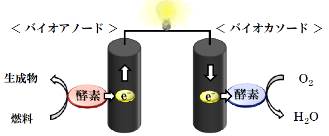
Bio-fuel cell is the fuel cell using enzyme reaction for redox reaction and saccharides, alcoholes and oxygen as fuel. Bio-fuel cell is expected to apply as the power source of implantable medical equipment or portable devices. We try to improve the performances of bio-fuel cell by designing enzyme-immobilized electrodes with molecular functional materials.
Electrochemical sensor
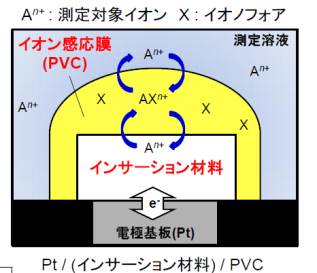
Electrochemical sensor is the device to quantify specific ions in solution, and widely used in the environmental and medical fields. We try to make ion sensors smaller and higher performance by applying insertion materials which we have studied for positive electrodes of rechargeable batteries. In addition, measurement condition of the glucose sensor is generally limited by the disadvantage of enzymes used for the glucose sensor that its enzymatic activities depend on pH and temperature. To solve this problem, we focus on the non-enzymatic glucose sensor, and enabled good glucose responses without enzymes by using metal oxides as electrode catalyst.
Movie